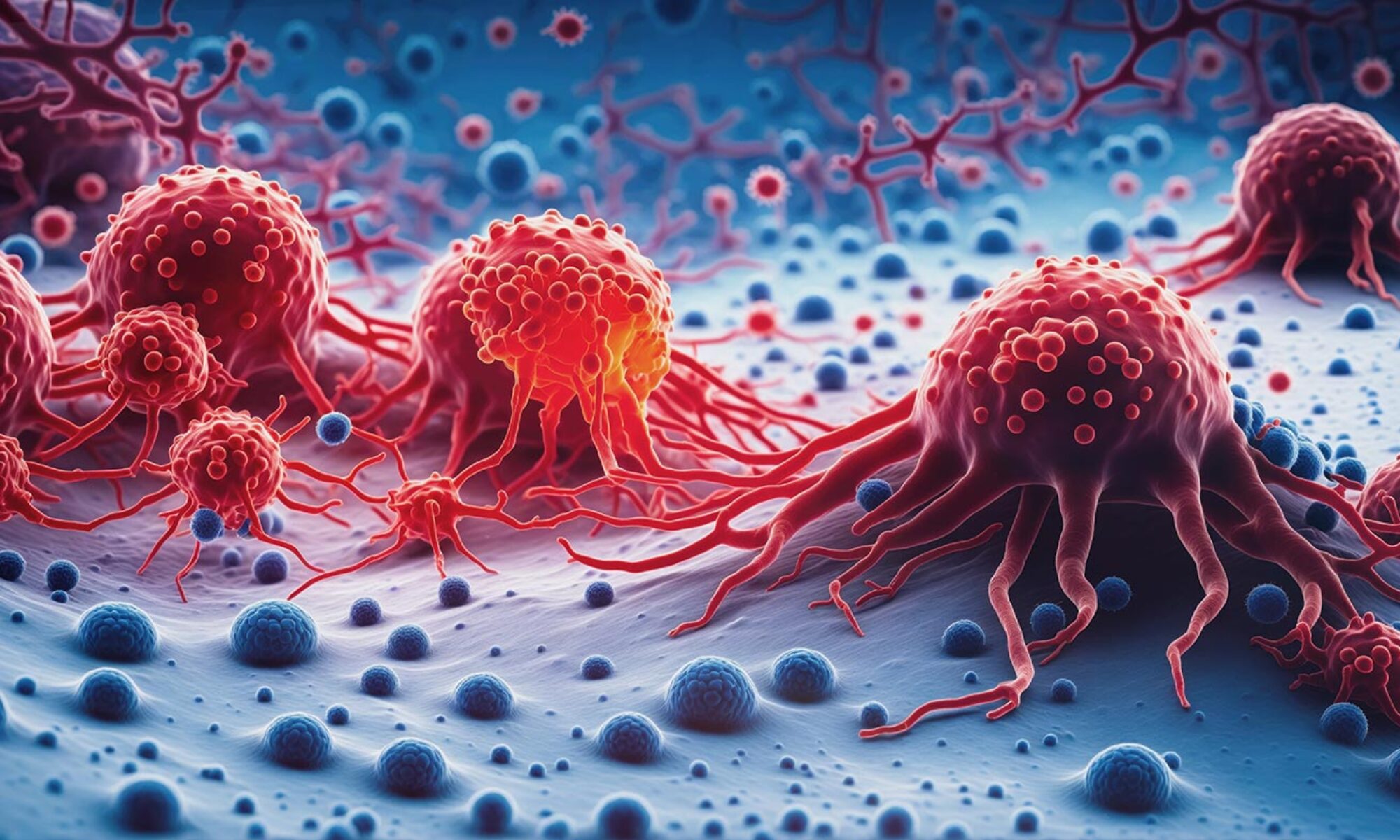
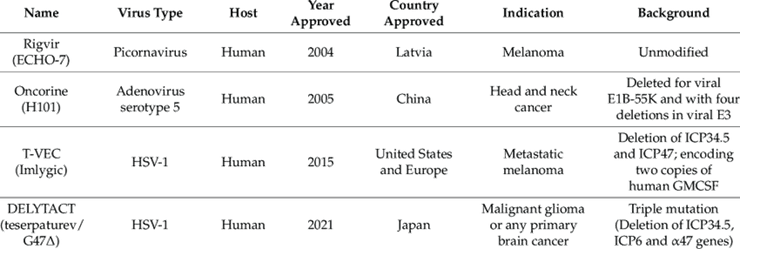
the approval was withdrawn in 2019. An oncolytic adenovirus, a genetically modified adenovirus named H101, was approved in China in 2005 for the treatment of head and neck cancer.[12] In 2015, talimogene laherparepvec (OncoVex, T-VEC), an oncolytic herpes virus which is a modified herpes simplex virus, became the first oncolytic virus to be approved for use in the United States and the European Union, for the treatment of advanced inoperable melanoma.[13]
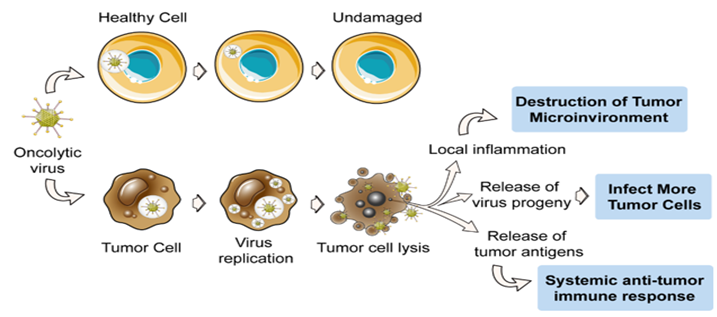
History
A connection between cancer regression and viruses has long
been theorised, and case reports of regression noted in cervical cancer,
Burkitt lymphoma, and Hodgkin lymphoma, after immunisation or infection with an
unrelated virus appeared at the beginning of the 20th century.[15] Efforts to
treat cancer through immunisation or virotherapy (deliberate infection with a
virus), began in the mid-20th century.[15][16] As the technology to create a
custom virus did not exist, all early efforts focused on finding natural
oncolytic viruses. During the 1960s, promising research involved using
poliovirus,[17] adenovirus,[15] Coxsackie virus,[18] ECHO enterovirus
RIGVIR,[19] and others.[16] The early complications were occasional cases of
uncontrolled infection (resulting in significant morbidity and mortality); an
immune response would also frequently develop. While not directly harmful to
the patient,[15] the response destroyed the virus thus preventing it from
destroying the cancer.[17] Early efforts also found that only certain cancers
could be treated through virotherapy.[18] Even when a response was seen, these
responses were neither complete nor durable.[15] The field of virotherapy was
nearly abandoned for a time, as the technology required to modify viruses
didn't exist whereas chemotherapy and radiotherapy technology enjoyed early
success. However, now that these technologies have been thoroughly developed
and cancer remains a major cause of mortality, there is still a need for novel
cancer therapies, garnering this once-sidelined therapy renewed
interest.[15][20] In one case report published in 2024, a scientist Beata
Halassy treated her own stage 3 breast cancer using an Edmonston-Zagreb measles
vaccine strain (MeV) and then a vesicular stomatitis virus Indiana strain
(VSV), both prepared in her own laboratory, in combination with trastuzumab.
While the treatment was successful and self-experimentation has a long history
in science, the decision to publish the case report attracted controversy due
to the unapproved nature of the viral agents and treatment protocol
used.[21][22]
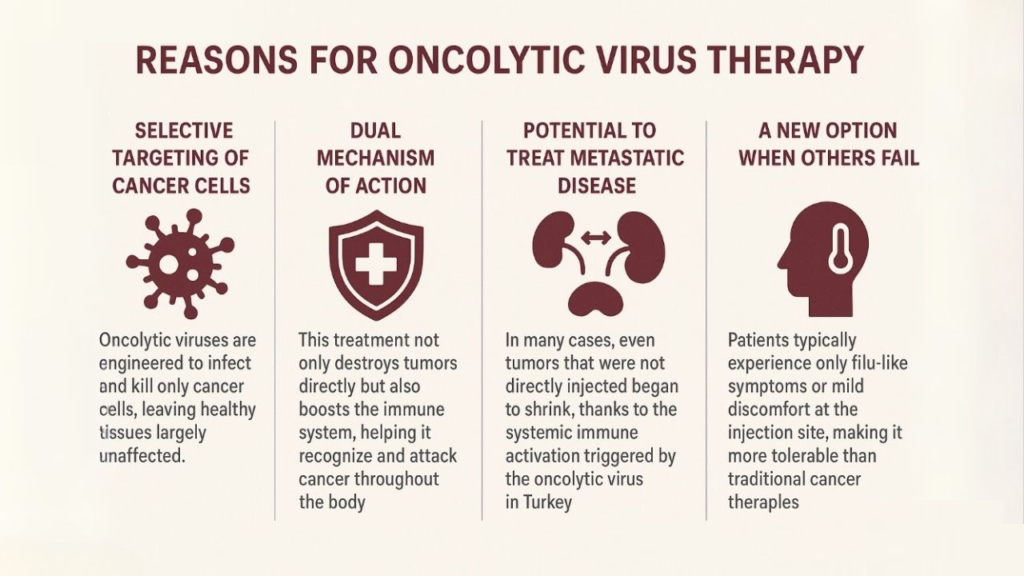
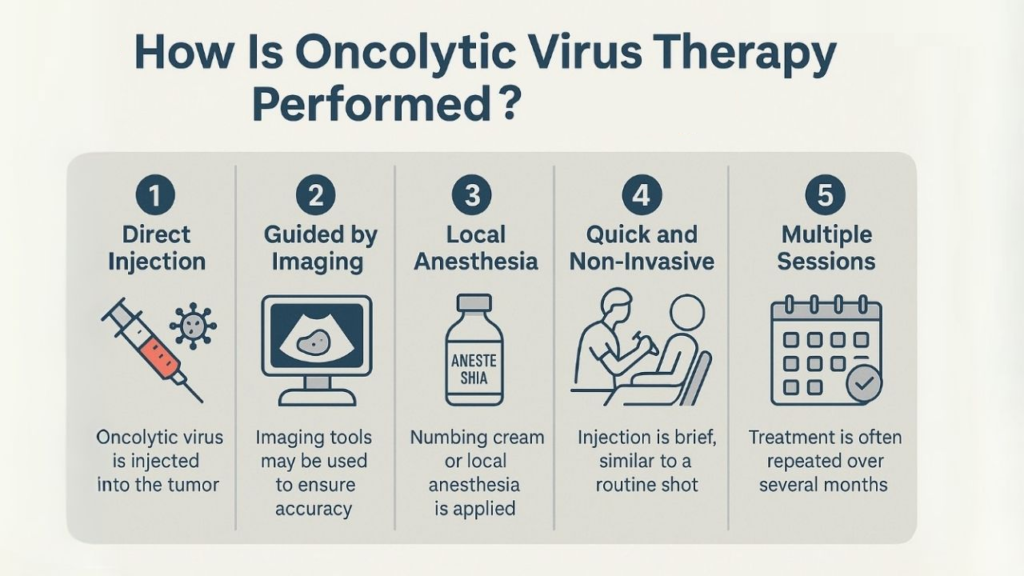
1-
Ferguson MS, Lemoine NR, Wang Y (2012). "Systemic delivery of oncolytic viruses: hopes and
hurdles". Advances in Virology. 2012: 1–14. doi:10.1155/2012/805629. PMC 3287020. PMID 22400027.
2.
Casjens S (2010). "Oncolytic virus". In Mahy BW, Van
Regenmortel MH (eds.). Desk Encyclopedia of General Virology. Boston:
Academic Press. p. 167. ISBN 978-0-12-375146-1.
3.
Melcher A, Parato K, Rooney CM, Bell JC (June 2011). "Thunder and lightning: immunotherapy and oncolytic
viruses collide". Molecular Therapy. 19 (6): 1008–16. doi:10.1038/mt.2011.65. PMC 3129809. PMID 21505424.
4.
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC (August 2014).
"Going viral with cancer immunotherapy". Nature Reviews.
Cancer. 14 (8): 559–67. doi:10.1038/nrc3770. PMID 24990523. S2CID 15182671.
5.
De Silva, Naomi; Atkins, Harold; Kirn, David H.; Bell, John C.;
Breitbach, Caroline J. (1 April 2010). "Double trouble for tumours: Exploiting the tumour
microenvironment to enhance anticancer effect of oncolytic viruses". Cytokine
& Growth Factor Reviews. Recent Advances in the Development of Oncolytic
Viruses as Cancer Therapeutics. 21 (2): 135–141. doi:10.1016/j.cytogfr.2010.02.007. ISSN 1359-6101. PMID 20338801.
6.
"Using Viruses to Treat Cancer | Science-Based
Medicine". sciencebasedmedicine.org. 28 September 2022.
Retrieved 4 November 2022.
7.
Alemany R (March 2013). "Viruses
in cancer treatment". Clinical & Translational
Oncology. 15 (3): 182–8. doi:10.1007/s12094-012-0951-7. PMID 23143950. S2CID 6123610.
8.
Donnelly OG, Errington-Mais F, Prestwich R, Harrington K, Pandha
H, Vile R, Melcher AA (July 2012). "Recent clinical experience with
oncolytic viruses". Current Pharmaceutical Biotechnology. 13 (9): 1834–41. doi:10.2174/138920112800958904. PMID 21740364.
9.
Roberts MS, Lorence RM, Groene WS, Bamat MK (August 2006).
"Naturally oncolytic viruses". Current Opinion in Molecular
Therapeutics. 8 (4): 314–21. PMID 16955694.
10.
Rudin CM, Poirier JT, Senzer NN, Stephenson J, Loesch D,
Burroughs KD, Reddy PS, Hann CL, Hallenbeck PL (February 2011). "Phase I clinical study of Seneca Valley Virus
(SVV-001), a replication-competent picornavirus, in advanced solid tumors with
neuroendocrine features". Clinical Cancer Research. 17 (4): 888–95. doi:10.1158/1078-0432.CCR-10-1706. PMC 5317273. PMID 21304001.
11.
"Rigvir šķīdums injekcijām". Medicinal
product register of the Republic of Latvia. 29 April 2004. Retrieved 8
December 2016.
12
Frew SE, Sammut SM, Shore AF, Ramjist JK, Al-Bader S, Rezaie R,
Daar AS, Singer PA (January 2008). "Chinese health biotech and the billion-patient
market". Nature Biotechnology. 26 (1): 37–53. doi:10.1038/nbt0108-37. PMC 7096943. PMID 18183014.
13
Broderick J (29 April 2015). "FDA Panels Support Approval of T-VEC in
Melanoma". OncLive. Retrieved 24 August 2015.
14.
Research, Center for Drug Evaluation and (29 December
2022). "FDA approves first adenoviral vector-based gene
therapy for high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive
bladder cancer". FDA. Archived from the original on 19 December 2022.
Kuruppu D, Tanabe KK (May 2005). "Viral
oncolysis by herpes simplex virus and other viruses". Cancer
Biology & Therapy. 4 (5): 524–31. doi:10.4161/cbt.4.5.1820. PMID 15917655.
16.
Voroshilova MK (1989).
"Potential use of nonpathogenic enteroviruses for control of human
disease". Progress in Medical Virology. Fortschritte der
Medizinischen Virusforschung. Progrès en Virologie Médicale. 36: 191–202. PMID 2555836.
17.
Pond AR, Manuelidis EE (August 1964). "Oncolytic Effect of Poliomyelitis Virus on Human
Epidermoid Carcinoma (Hela Tumor) Heterologously Transplanted to Guinea
Pigs". The American Journal of Pathology. 45 (2): 233–49. PMC 1907181. PMID 14202523.
18.
Kunin CM (December 1964). "Cellular Susceptibility to Enteroviruses". Bacteriological
Reviews. 28 (4): 382–90. doi:10.1128/MMBR.28.4.382-390.1964. PMC 441234. PMID 14244713.
19.
Chumakov PM, Morozova VV, Babkin IV, Baĭkov IK, Netesov SV,
Tikunova NV (2012). "[Oncolytic enteroviruses]". Molekuliarnaia
Biologiia (in Russian). 46 (5): 712–25. doi:10.1134/s0026893312050032. PMID 23156670. S2CID 3716727.
20.
Kelly E, Russell SJ (April 2007). "History
of oncolytic viruses: genesis to genetic engineering". Molecular
Therapy. 15 (4): 651–9. doi:10.1038/sj.mt.6300108. PMID 17299401.
21.
Forcic
D, et al. An Unconventional Case Study of Neoadjuvant Oncolytic Virotherapy for
Recurrent Breast Cancer. Vaccines 2024; 12(9): 958. doi:10.3390/vaccines12090958
22.
Corbyn
Z. This scientist treated her own cancer with viruses she grew in the
lab. Nature News 2024 doi:10.1038/d41586-024-03647-0